Cyto3D Live-Dead Assay Kit
| Specifications | |
|---|---|
| Product Category: | Cell Proliferation and Viability Assays |
| Detection Method: | Fluorescence |
| Assay Category: | Staining Reagents |
| Sample Type: | Cells/organoids cultured in 3D, 2D coating, and on monolayer |
Product Description
The Cyto3D® Live-Dead Assay Kit is used to determine the live/dead nucleated cells by using a fast one-step staining procedure for analysis on a dual-fluorescence system. This kit is recommended for viability analysis of cells/organoids cultured in 3D, 2D coating, and on monolayer.
Acridine orange (AO) and propidium iodide (PI), both nuclear staining (nucleic acid binding) dyes, are used in this kit. AO is permeable to both live and dead cells and stains all nucleated cells to generate green fluorescence. PI only penetrates the membranes of nucleated cells with compromised membranes and stains the dead cells to generate red fluorescence. Due to the quenching, when cells are stained with both AO and PI, all live nucleated cells fluoresce green and all dead nucleated cells fluoresce red (the PI reduces the fluorescence intensity of the AO by fluorescence resonance energy transfer (FRET)). Non-nucleated materials such as red blood cells, platelets and debris do not fluorescence and are ignored fluorescence microscopes.
Dual-Fluorescence Viability, using AO and PI, is the recommended viability analysis method for cell lines, primary cells, and stem cells.
Related Products
VitroGel® Xeno-free Functional Hydrogel System
Easy setup and use
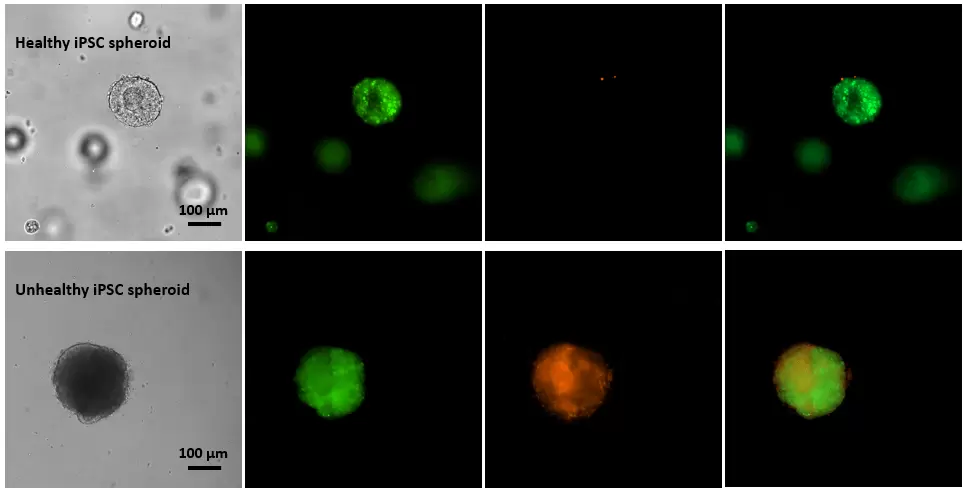
Figure 1. Live-dead cell viability images of stem cell spheroids.
Stem cells were static suspension culture in VitroGel STEM (cat# VHM02) for 5 days. 2 µL of Cyto3D reagent was added to each well containing 100 µL cell suspension. The mixture was incubated at 37 °C for 5-10 min. The cells were then observed under a fluorescent microscope. The images show the Live (green) and Dead (orange) stem cell spheroids cultured in a 3D hydrogel matrix. The live-dead dyes of Cyto3D Live-Dead Assay Kit can successfully penetrate into the big cell spheroids for cell viability analysis.
Application Notes
- Long Term 3D Tumor Spheroid Culture in VitroGel Hydrogel Matrix
- Advanced 3D Luminal Breast Cancer Model in VitroGel® System: Long-term 3D Cell Culture and Co-culture with Fibroblast Cells
- Advanced Skin Cell Models Using the VitroGel® System: 2D Coating and 3D Cell Culture with Human Dermal Fibroblasts
Product Citations
- Babl, N., Hofbauer, J., Matos, C., Voll, F., Ayse Nur Menevse, Rechenmacher, M., Mair, R., Philipp Beckhove, Herr, W., Siska, P. J., Renner, K., Kreutz, M., & Schnell, A. (2023) Low-density lipoprotein balances T cell metabolism and enhances response to anti-PD-1 blockade in a HCT116 spheroid model Frontiers in Oncology
- Belén, A., Sacconi, A., Tremante, E., Lulli, V., Caprara, V., Rosanò, L., Goeman, F., Carosi, M., Marta Di Giuliani, Vari, G., Silvani, A., Pollo, B., Garufi, C., Ramponi, S., Simonetti, G., Ciusani, E., Chiara Mandoj, Stefano Scabini, Villani, V., & Agnese Pò. (2023) A diagnostic circulating miRNA signature as orchestrator of cell invasion via TKS4/TKS5/EFHD2 modulation in human gliomas Journal of Experimental & Clinical Cancer Research
- Wan, Y., Zhang, Y., Meng, H., Miao, H., Jiang, Y., Zhang, L., & Cheng, W (2023) Bractoppin, a BRCA1 carboxy-terminal domain (BRCT) inhibitor, suppresses tumor progression in ovarian borderline tumor organoids BBRC
- (2021) Mitogen-activated protein kinase activity drives cell trajectories in colorectal cancer EMBO Mol Med
- Di Donato, M., Galasso, G., Giovannelli, P., Sinisi, A. A., Migliaccio, A., & Castoria, G. (2021) Targeting the Nerve Growth Factor Signaling Impairs the Proliferative and Migratory Phenotype of Triple-Negative Breast Cancer Cells Frontiers in Cell and Developmental Biology
- Catalog Number
BM01-TW - Supplier
TheWell - Size
- Shipping
RT

